Research in the Dempsey group aims to address challenges associated with developing efficient solar energy conversion processes. We are particularly interested in charge transfer processes associated with solar fuel production, including proton-coupled electron transfer reactions and electron transfer across interfaces. Our research program bridges molecular and materials chemistry and relies heavily on methods of physical inorganic chemistry, including transient absorption spectroscopy and electrochemistry.
Proton-Coupled Electron Transfer
Central to photosynthesis and solar fuel production is a process known as proton-coupled electron transfer (PCET). PCET is a fundamental charge transfer reaction wherein the reaction pathways of protons and electrons are intimately coupled. Solar fuel-generating reactions rely on this fundamental process, as both protons and electrons must be managed for light-driven water splitting. Revealing exactly how PCET proceeds in systems like these is vital for advancing sustainable energy technologies.
In one project, we interrogate the pathways by which molecular transition metal-based catalysts mediate the electrochemical reduction of protons to hydrogen. Specifically, how do these coordination complexes choreograph the movement of protons and electrons to generate fuels? What parameters influence the mechanisms of reactivity? Can the system be tuned to promote a concerted reaction pathway? Can we apply this knowledge to design better catalysts? To answer these questions, we combine inorganic synthesis, electrochemical techniques, and time-resolved spectroscopies to measure electron and proton transfer kinetics, detect intermediates, and resolve the mechanisms by which these catalysts operate.
In a second project, we are interested in discovering how light absorption can be coupled to proton-coupled electron transfer processes. By integrating the capture of solar photons with fuel-forming PCET reactions, we hope to realize new ways by which solar energy can be utilized to directly drive the production of fuels. To accomplish this, we are using a combination of inorganic synthesis and photochemistry to interrogate how electronic structure can be used to influence PCET reactivity. The details revealed by these studies will ultimately provide a better understanding of the role PCET plays in solar fuel producing reactions and allow us to harness light-driven PCET reactions for energy conversion.
Liquid Solar Fuels
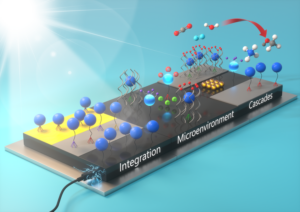
Driving the production of liquid fuels from carbon dioxide and water with sunlight represents an exciting strategy to produce storable fuels directly from a renewable energy source. By pairing the light-absorbing properties of semiconductor materials with molecular catalysts that selectively produce fuels, the Center for Hybrid Approaches in Solar Energy to Liquid Fuels (CHASE), an DOE Fuels from Sunlight Energy Innovation Hub is advancing the science of liquid solar fuels. Together with our collaborators in CHASE, we are studying the integration of molecular catalysts that mediate reduction of carbon dioxide with silicon semiconductors to access hybrid photoelectrodes.
Chemistry in an Optical Cavity
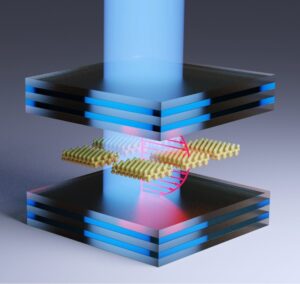
Quantum light-matter interactions between molecules and radiation inside an optical cavity present a unique opportunity to enable new chemical reactivities. As part of the Center for Quantum Electrodynamics for Selective Transformations (QuEST), we are developing strategies for making electrochemical measurements inside an optical cavity.
Interfacial Electron Transfer
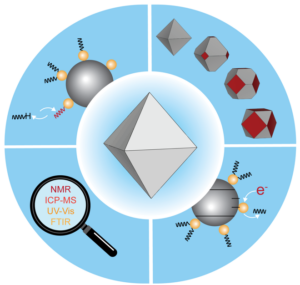
Interfacial electron transfer processes dictate efficiency in devices proposed for energy capture and conversion. To help realize new nanoparticle-based materials for energy capture and conversion, our lab studies the semiconductor nanocrystal surfaces and how its properties influence electron transfer processes across the nanocrystal interface. We explore the composition of semiconductor quantum dot surfaces and probe their reactivity using a combination of NMR, UV-Vis absorption spectroscopy, inductively-coupled plasma mass spectrometry, and luminescence spectroscopy. We also study how the addition of charge carriers to these materials (doping) influences their surface composition and are employ a suite of physical methods to reveal the mechanisms of doping.